
This means it will no longer participate in the chemical reaction so the battery needs to repaired or replaced. If we were to leave the battery to fully discharge for too long, or too many times- it becomes very difficult to reverse the chemical reaction.Īdditionally, the sulphate layer could break away from the electrodes and accumulate at the bottom of the battery.

The oxygen ions combined with the lead to create lead oxide and this releases the sulphate back into the electrolyte making it even more stronger. The sulphate ions enter the electrolyte and combined with the hydrogen ions to release the oxygen ions, and so the electrolyte acid becomes stronger.
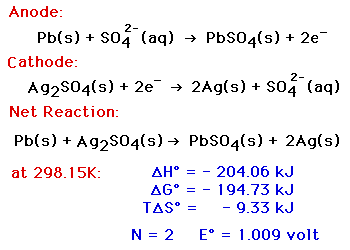
The electrons enter the negative terminal and re-join with the lead sulphate, releasing the sulphate into the electrolyte to leave just lead on the negative plate. So if we supply the battery with electricity from the alternator, we can start to reverse the reaction. But luckily this chemical reaction can be reversed. This means the materials of the electrodes are becoming more similar and so the chemical reaction becomes harder to achieve. The chemicals required for the reaction will run out, the acid becomes diluted and weaker and a build-up of lead sulphate coats both of the electrodes. While the path exists the chemical reaction continues, but this wont last forever. We can then place things such as a lamp in the way of these and use them to do work such as illuminating the lamp. So, if we provide a path for the electrons, such as wire, then the electrons will flow through this to get the positive terminal. These are attracted to the positive terminal which has less electrons, however, they cant reach this yet. If you think about a magnet, the electrons are negatively charged and they repel each other. And we can measure this with a voltmeter or a multimeter. As electrons are negatively charged this means we have a difference in charge across the two terminals. So now we have a build up of electrons on the negative terminal. During this reaction two electrons are released and collected in the negative terminal. This reaction will create a layer of lead sulphate around the electrode. At the same time the lead atoms on the anode are going to react with the sulphate ions in the electrolyte. Once in the electrolyte these oxygen ions will combine with the hydrogen ions to form water. During this reaction an oxygen ion is ejected from the cathode and into the electrolyte. This will form a layer of lead sulphate on the cathode terminal. The positive cathode terminal of lead oxide is going to react with the sulphate in the electrolyte. I’ll show the atoms of these materials with these coloured spheres. When these materials are combined we’re going to get a small chemical reaction between the atoms. We then have the negative terminal which is the anode. We have the positive electrode which is the cathode. In this cell we have the electrolyte liquid, which is one-third sulphuric acid and two-thirds water. Rather than trying to understand this complex construction, we are going to simplify it down to this simple model of a cell with a single cathode and anode. If the atom has more protons than electrons, then the positive ion. If the atom has more electrons than protons then it’s a negative ion. An atom has a neutral charge when it has the same number of protons and electrons because the protons are positively charged and the electrons are negatively charged so they balance out. An ion is an atom which has an unequal number of protons or electrons. When we talk about atoms you’ll usually hear the term ion used. Electrons can also be released or captured by atoms during this reaction.

During this interaction atoms will bond together or break apart. This is when the atoms of one material interact with the atoms of another material. When we mix certain chemicals together we can cause chemical reactions.

Scroll to the bottom to watch the YouTube tutorial. We’ll cover the basics of lead acid batteries, including their composition and how they work. In this article, we’re going to learn about lead acid batteries and how they work.


 0 kommentar(er)
0 kommentar(er)
